Difference between revisions of "PARCLIP data analysis using CTK"
From Zhang Laboratory
(Created page with "<center>CTK home | Standard/BrdU-CLIP | iCLIP | [[eCLIP data analysis using CTK|eCLIP]...") |
|||
| Line 516: | Line 516: | ||
One can repeat these steps for the other types of mutations (i.e. deletions, insertions and general substitutions). This is particularly relevant when one works with a new RBP, and it is unknown which type of mutations will be caused by cross linking. | One can repeat these steps for the other types of mutations (i.e. deletions, insertions and general substitutions). This is particularly relevant when one works with a new RBP, and it is unknown which type of mutations will be caused by cross linking. | ||
| + | |||
| + | |||
| + | =Motif enrichment= | ||
| + | |||
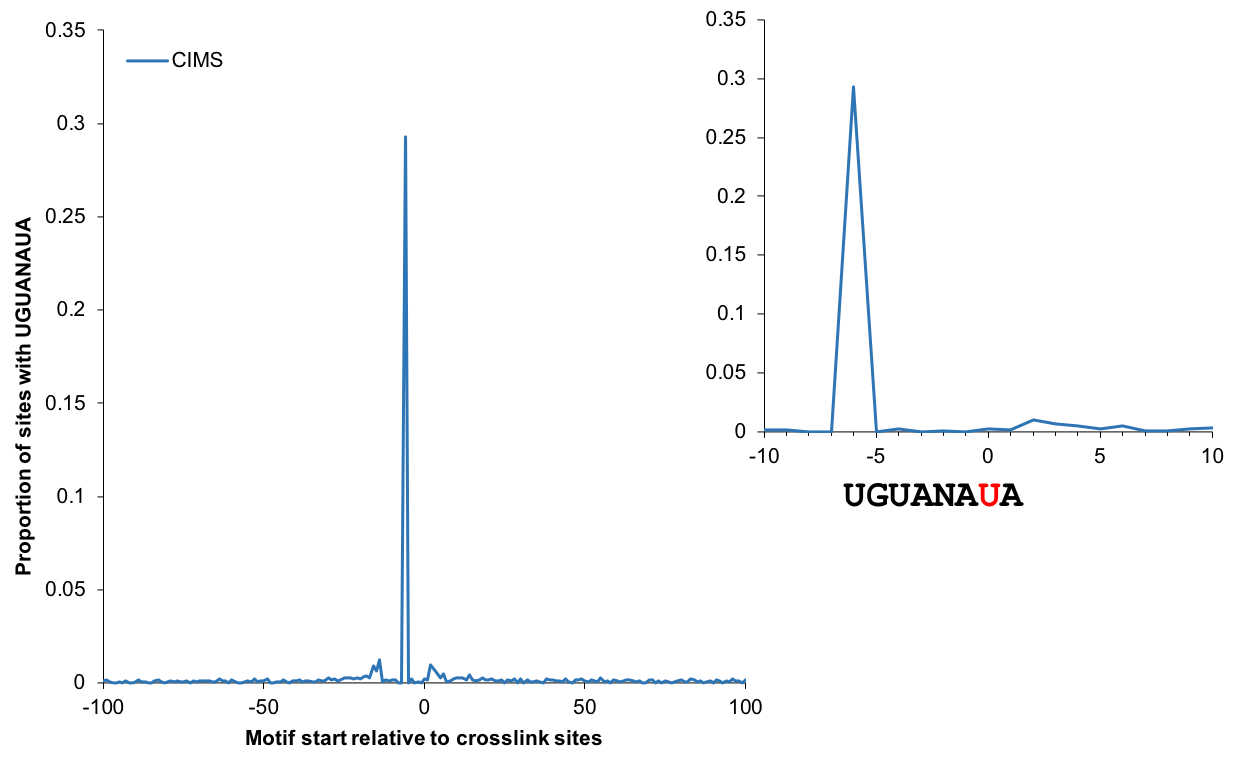
| + | The enrichment of motif sites (when the motif is known) around CIMS or CITS provides a quantitative measure of the signal to noise ratio. The plot below shows the proportion of sites with the PUM2 binding UGUANAUA motif starting at each position relative to CIMS (T-C substitutions). This analysis shows U7 as the major crosslink sites in this dataset, which is consistent with the analysis of the original study (Hafner et al. 2010). | ||
| + | |||
| + | <html> | ||
| + | <img src="/data/CTK/PARCLIP_PUM2.png" width="100%"></img> | ||
| + | |||
| + | </html> | ||
Latest revision as of 16:49, 24 May 2018
Contents
Introduction
This tutorial outlines how to analyze CLIP data derived from the Photoactivatable-Ribonucleoside-Enhanced Crosslinking and Immunoprecipitation (PAR-CLIP) protocol. The method relies on the incorporation of photoreactive ribonucleoside analogs, like 4-thiouridine (4-SU) and 6-thioguanosine (6-SG), into nascent RNA transcripts. Irradiation of cells by UV light crosslinks the photoreactive nucleoside-labeled cellular RNAs to interacting RNA binding proteins.
One characteristic feature of cDNA libraries prepared by PAR-CLIP is that crosslinking can be identified by mutations in the sequenced cDNA. 4-SU induces a thymidine to cytidine (T->C) transition, whereas 6-SG induces guanosine to adenosine (G->A) mutations.
Hafner, M., Landthaler, M., Burger, L., Khorshid, M., Hausser, J., Berninger, P., ... & Ulrich, A. (2010). PAR-CliP-a method to identify transcriptome-wide the binding sites of RNA binding proteins. JoVE (Journal of Visualized Experiments), (41), e2034-e2034.
PAR-CLIP reads typically have the following structure:
[CLIP tag sequence]TCGTATGCCGTCTTCTGCTTG
Sample dataset
We will use PUM2 PAR-CLIP data from 293T cells generated by Hafner et al. (2010) for this tutorial.
Raw FASTQ files
We will use ~/test as our working directory, and download fastq files under ~/test/fastq
mkdir ~/test; cd ~/test mkdir fastq; cd fastq
The raw sequence files from Illumina sequencing can be downloaded from the ENCODE web site.
wget ftp://ftp-trace.ncbi.nih.gov/sra/sra-instant/reads/ByRun/sra/SRR/SRR048/SRR048967/SRR048967.sra wget ftp://ftp-trace.ncbi.nih.gov/sra/sra-instant/reads/ByRun/sra/SRR/SRR048/SRR048968/SRR048968.sra #convert to fastq files fastq-dump -F --gzip SRR048967.sra fastq-dump -F --gzip SRR048968.sra ln -s SRR048967.fastq.gz PUM2_high.fastq.gz ln -s SRR048968.fastq.gz PUM2_low.fastq.gz
Note that two bands of high and low molecular weight were cut out and used to prepare independent PAR-CLIP libraries. These were treated biological replicates in the original study and in this tutorial.
Output files of major steps generated in this tutorial
- Fully preprocessed FASTQ files (60 MB) : output files generated for use right before mapping.
- Unique CLIP tag and mutation files (12 MB) : output files generated after mapping and collapsing PCR duplicates.
- Peak calling files (691 KB) : output files after peak calling.
- CIMS output files (4 KB) : output files after CIMS analysis.
The number of reads in each file, after each step of processing, is summarized in Table 1 below.
| Protein | Sample | # of raw reads | # of trimmed & filtered reads | # of collapsed reads | # of mapped reads | # of unique tags |
|---|---|---|---|---|---|---|
| PUM2 | high | 5,104,559 | 4,477,899 | 1,053,740 | 81,310 | 30,539 |
| PUM2 | low | 5,351,797 | 3,495,032 | 1,058,679 | 198,223 | 129,781 |
| Total | 10,456,356 | 7,972,931 | 2,112,419 | 279,533 | 160,320 |
Read preprocessing
Read quality filtering
cd ~/test mkdir filtering cd filtering
The fastq_filter.pl script will extract reads passing quality filters for each library (of standard CLIP-seq data) using a simple loop.
#!/bin/bash for f in PUM2_high PUM2_low; do perl /usr/local/CTK/fastq_filter.pl -v -if sanger -f mean:0-24:20 -of fastq ../fastq/$f.fastq.gz - | gzip -c > $f.filter.fastq.gz done
Trimming of 3' linker sequences
cd ~/test mkdir filtering cd filtering
One can then trim the 3' adapter using the command below.
#!/bin/bash for $f in PUM2_high PUM2_low; do cutadapt -f fastq --times 1 -e 0.1 -O 1 --quality-cutoff 5 -m 15 -a TCGTATGCCGTCTTCTGCTTG -o $f.filter.trim.fastq.gz $f.filter.fastq.gz > $f.cutadpt.log done
The parameters are as follows:
- -f fastq : input is a FASTQ file
- --times 1 : removes up to 1 adapters from each read; here we specify 2 as occasionally some tags have 2 copies of adapters
- -e 0.1 : maximum allowed error rate is 0.1
- -O 1 : the read is not modified if the overlap between the read and the adapter is shorter than 1nt
- --quality-cutoff : refers to quality
- -m 15 : minimum length of the read being 15nt tags
- -a : the 3' adapter
For more cutadapt usage information:
cutadapt --help
This is the same thing, but using a Sun Grid Engine to execute this UNIX job on a remote machine.
#!/bin/bash
#$ -t 1-2 -m a -cwd -N CLIP
files=(PUM2_high PUM2_low);
f=${files[$SGE_TASK_ID-1]}
cutadapt -f fastq --times 1 -e 0.1 -O 1 --quality-cutoff 5 -m 15 -a TCGTATGCCGTCTTCTGCTTG -o $f.filter.trim.fastq.gz $f.filter.fastq.gz > $f.cutadpt.log
Note 1: It is good to check the number of reads by running the following command:
#e.g., for raw reads
for f in `ls ../fastq/*.fastq.gz`; do
c=`zcat $f | wc -l`
c=$((c/4))
echo $f $c
done
#e.g., for trimmed reads
for f in `ls *.filter.trim.fastq.gz`; do
c=`zcat $f | wc -l`
c=$((c/4))
echo $f $c
done
Collapse Exact PCR Duplicates
Collapse exact PCR duplicates such that if multiple reads have exactly the same sequence, only one is kept.
#!/bin/bash -l
for f in PUM2_high PUM2_low; do
perl /usr/local/CTK/fastq2collapse.pl $f.filter.trim.fastq.gz - | gzip -c > $f.filter.trim.c.fastq.gz
done
Note 1: It is good to check the number of reads by running the command:
for f in `ls *.filter.trim.c.fastq.gz`; do
c=`zcat $f | wc -l`
c=$((c/4))
echo $f $c
done
Note 2: Get the distribution of tag lengths for diagnostic purposes using the following command.
for f in PUM2_high PUM2_low; do
zcat $f.filter.trim.c.fastq.gz | awk '{if(NR%4==2) {print length($0)}}' | sort -n | uniq -c | awk '{print $2"\t"$1}' > $f.filter.trim.c.seqlen.stat.txt
done
Download fully preprocessed FASTQ files here (60 MB).
Read mapping & parsing
We assume the reference index has been prepared and is available under /genomes/hg19/bwa/.
cd ~/test mkdir mapping cd mapping
Run bwa to align the reads to the reference genome.
for f in PUM2_high PUM2_low; do bwa aln -t 4 -n 0.06 -q 20 /genomes/hg19/bwa/hg19.fa ../filtering/$f.filter.trim.c.fastq.gz > $f.sai bwa samse /genomes/hg19/bwa/hg19.fa $f.sai ../filtering/$f.filter.trim.c.fastq.gz | gzip -c > $f.sam.gz done
The option -n 0.06 specifies slightly more stringent criteria than the default. The number of allowed mismatches (substitutions or indels) depending on read length is as follows:
[bwa_aln] 17bp reads: max_diff = 1 [bwa_aln] 20bp reads: max_diff = 2 [bwa_aln] 45bp reads: max_diff = 3 [bwa_aln] 73bp reads: max_diff = 4 [bwa_aln] 104bp reads: max_diff = 5 [bwa_aln] 137bp reads: max_diff = 6 [bwa_aln] 172bp reads: max_diff = 7 [bwa_aln] 208bp reads: max_diff = 8 [bwa_aln] 244bp reads: max_diff = 9
The -q option is used to trim low quality reads (an average of 20 or below). However, this does not seem to do too much after the trimming steps above.
Parsing SAM file
for f in PUM2_high PUM2_low; do perl /usr/local/CTK/parseAlignment.pl -v --map-qual 1 --min-len 18 --mutation-file $f.mutation.txt $f.sam.gz - | gzip -c > $f.tag.bed.gz done
This will keep only unique mappings (with MAPQ >=1) and a minimal mapping size of 18 nt.
Note 1: In the tag bed file, the 5th column records the number of mismatches (substitutions) in each read
Note 2: Other aligners might not use a positive MAPQ as an indication of unique mapping.
Another useful option is --indel-to-end, which specifies the number of nucleotides towards the end from which indels should not be called (default=5 nt).
Note 3: The parsing script relies on MD tags, which is an optional field without strict definition in SAM file format specification. Some aligners might have slightly different format how they report mismatches. If other aligners than bwa is used, one should run the following command:
samtools view -bS $f.sam | samtools sort - $f.sorted samtools fillmd $f.sorted.bam /genomes/hg19/bwa/hg19.fa | gzip -c > $f.sorted.md.sam.gz
This will ensure the sam file gets parsed properly.
Note 4: Keep track what proportion of reads can be mapped uniquely.
wc -l *.tag.bed
Collapse PCR duplicates
It is critical to collapse PCR duplicates, not only for the exact duplicates collapsed above, but also for those with slight differences due to sequencing errors.
for f in PUM2_high PUM2_low; do perl /usr/local/CTK/tag2collapse.pl -v -big -weight --weight-in-name --keep-max-score --keep-tag-name $f.tag.bed $f.tag.uniq.bed done
In the command above, tags mapped to the same genomic positions (i.e., the same start coordinates for the 5' end of RNA tag) will be collapsed together. Note that this PAR-CLIP dataset does not have random barcode attached CLIP tags that can be used as unique molecular identifiers (UMIs).
Note 1: Note that the read ID in the input BED file (in the 4th column) must take the form READ#x, where x is the number of exact duplicates. Read IDs that are not in this format will generate an error.
Note 2: As a diagnostic step, get the length distribution of unique tags, which should be a more faithful representation of the library:
for f in PUM2_high PUM2_low; do
awk '{print $3-$2}' $f.tag.uniq.bed | sort -n | uniq -c | awk '{print $2"\t"$1}' > $f.tag.uniq.len.dist.txt
done
Get the mutations in unique tags.
for f in PUM2_high PUM2_low; do python /usr/local/CTK/joinWrapper.py $f.mutation.txt $f.tag.uniq.bed 4 4 N $f.tag.uniq.mutation.txt done
This script is from galaxy, and included for your convenience. The parameters 4 4 indicate the columns in the two input file used to join the two, and N indicates that only paired rows should be printed.
Table 2 summarizes the number of unique mutations of different types in each sample.
| Protein | Sample | # of unique tags | Total # of mutations in unique tags | Deletions | Insertions | Substitutions | T2C |
|---|---|---|---|---|---|---|---|
| PUM2 | high | 30,539 | 30,467 | 2,187 | 1,086 | 27,194 | 17,557 |
| PUM2 | low | 129,781 | 147,772 | 5,102 | 15,019 | 127,651 | 46,302 |
| Total | 160,320 | 178,239 | 7,289 | 16,105 | 154,845 | 63,859 |
Merging biological replicates
After getting unique tags, one might want to concatenate these runs, which can be distinguished by different colors. As an example:
perl /usr/local/CTK/bed2rgb.pl -v -col "188,0,0" PUM2_high.tag.uniq.bed PUM2_high.tag.uniq.rgb.bed perl /usr/local/CTK/bed2rgb.pl -v -col "128,0,128" PUM2_low.tag.uniq.bed PUM2_low.tag.uniq.rgb.bed
Then these can be concatenated.
cat PUM2_high.tag.uniq.rgb.bed PUM2_low.tag.uniq.rgb.bed> PUM2.pool.tag.uniq.rgb.bed cat PUM2_high.tag.uniq.mutation.txt PUM2_low.tag.uniq.mutation.txt > PUM2.pool.tag.uniq.mutation.txt
Download unique tag and mutation files here (12 MB).
Annotating and visualizing CLIP tags
Get the genomic distribution of CLIP tags
perl /usr/local/CTK/bed2annotation.pl -dbkey hg19 -ss -big -region -v -summary PUM2.pool.tag.uniq.annot.summary.txt PUM2.pool.tag.uniq.rgb.bed PUM2.pool.tag.uniq.annot.txt
Make sure the current genome (hg19) is specified. Check the summary file (PUM2.pool.tag.uniq.annot.summary.txt) for the percentage of tags mapped to CDS, 3'UTR, introns, etc.
Generate bedgraph for visualization in the genome browser
perl /usr/local/CTK/tag2profile.pl -v -ss -exact -of bedgraph -n ″Unique Tag Profile″ PUM2.pool.tag.noRNA.uniq.rgb.bed PUM2.pool.tag.uniq.bedgraph
The output bedgraph file can be loaded into a genome browser for visualization of tag number at each genomic position.
Peak calling
cd ~/test mkdir cluster cd cluster ln -s ../mapping/PUM2.pool.tag.uniq.rgb.bed
Mode 1: Peak calling with no statistical significance
perl /usr/local/CTK/tag2peak.pl -big -ss -v --valley-seeking --valley-depth 0.9 PUM2.pool.tag.uniq.rgb.bed PUM2.pool.tag.uniq.peak.bed --out-boundary PUM2.pool.tag.uniq.peak.boundary.bed --out-half-PH PUM2.pool.tag.uniq.peak.halfPH.bed
Note 1: To annotate peaks with overlapping genes and repeat masked sequences and get genomic breakdown:
perl /usr/local/CTK/bed2annotation.pl -dbkey hg19 -ss -big -region -v -summary PUM2.pool.tag.uniq.peak.annot.summary.txt PUM2.pool.tag.uniq.peak.bed PUM2.pool.tag.uniq.peak.annot.txt
The output file PUM2.pool.tag.uniq.peak.annot.txt has the detailed annotation for each peak, and PUM2.pool.tag.uniq.peak.annot.summary.txt has summary statistics.
Note 2: Another useful option --valley-depth specifies the depth of the valley relative to the peak (0.5-1, 0.9 by default). One also has the option to merge peaks close to each other by specifying the distance between the peaks (e.g., -gap 20).
Note 3: Besides the peak boundaries (PUM2.pool.tag.uniq.peak.bed), one can also output cluster boundaries (--out-boundary) and half peak boundaries (--out-half-PH ) associated with each peak.
In this mode, the script will not assess the statistical significance of the peak height. If one needs this, an alternative mode is as follows:
Mode 2: Peak calling with statistical significance
perl /usr/local/CTK/tag2peak.pl -big -ss -v --valley-seeking -p 0.05 --valley-depth 0.9 --multi-test --dbkey hg19 PUM2.pool.tag.uniq.rgb.bed PUM2.pool.tag.uniq.peak.sig.bed --out-boundary PUM2.pool.tag.uniq.peak.sig.boundary.bed --out-half-PH PUM2.pool.tag.uniq.peak.sig.halfPH.bed
Note 1: To get the number of significant peaks:
wc -l PUM2.pool.tag.noRNA.uniq.peak.sig.bed 2,684 PUM2.pool.tag.uniq.peak.sig.bed
Note 2: To search for a known binding motif, one first defines the center of each peak (based on width at peak).
#extract from -500 to +500
awk '{print $1"\t"int(($2+$3)/2)-500"\t"int(($2+$3)/2)+500"\t"$4"\t"$5"\t"$6}' PUM2.pool.tag.uniq.peak.sig.bed > PUM2.pool.tag.uniq.peak.sig.center.ext1k.bed
This bed file can be used to extract sequences around peak (pay attention to the strand), and search for enrichment of specific motif (e.g., UGUANAUA) relative to the peak.
Note 3: One might want to count the number of tags overlapping with each cluster/peak for each sample (e.g., to evaluate correlation between replicates).
for f in HepG2.RBFOX2.rep1.R2 HepG2.RBFOX2.rep2.R2; do
perl /usr/local/CTK/tag2profile.pl -ss -region PUM2.pool.tag.uniq.peak.sig.boundary.bed -of bed -v $f.tag.uniq.bed $f.tag.uniq.peak.sig.boundary.count.bed
done
Download output files for peak calling here (691 KB).
CIMS
cd ~/test mkdir CIMS cd CIMS ln -s ../mapping/PUM2.pool.tag.uniq.rgb.bed ln -s ../mapping/PUM2.pool.tag.uniq.mutation.txt
Get specific types of mutations
Get specific types of mutations, such as deletions, substitutions, and insertions in unique CLIP tags
perl ~/czlab_src/CTK/getMutationType.pl -t del PUM2.pool.tag.uniq.mutation.txt PUM2.pool.tag.uniq.del.bed #deletions perl ~/czlab_src/CTK/getMutationType.pl -t ins PUM2.pool.tag.uniq.mutation.txt PUM2.pool.tag.uniq.ins.bed #insertions perl ~/czlab_src/CTK/getMutationType.pl -t sub PUM2.pool.tag.uniq.mutation.txt PUM2.pool.tag.uniq.sub.bed #substitutions perl ~/czlab_src/CTK/getMutationType.pl -t t2c --summary PUM2.pool.tag.uniq.mutation.stat.txt PUM2.pool.tag.uniq.mutation.txt PUM2.pool.tag.uniq.t2c.bed #t2c substitutions, as well as summary statistics of different types of mutations
It is always a good practice to look at the number of each type of mutation to see, e.g., compare the relative abundance of deletions to insertions. This information is provided in the summary statistics file when one specify the option --summary.
Get CIMS
Here, we use deletions as an example.
perl /usr/local/CTK/CIMS.pl -big -n 10 -p -outp PUM2.pool.tag.uniq.t2c.pos.stat.txt -v PUM2.pool.tag.uniq.rgb.bed PUM2.pool.tag.uniq.t2c.bed PUM2.pool.tag.uniq.t2c.CIMS.txt
By default, CIMS.pl will output all sites including those that are not statistically significant. This is recommended because one can play with stringency later.
Extract sites that are statistically significant.
awk '{if($9<=0.05) {print $0}}' PUM2.pool.tag.uniq.t2c.CIMS.txt | sort -k 9,9n -k 8,8nr -k 7,7n > PUM2.pool.tag.uniq.t2c.CIMS.s13.txt
cut -f 1-6 PUM2.pool.tag.uniq.t2c.CIMS.s13.txt > PUM2.pool.tag.uniq.t2c.CIMS.s13.bed
wc -l PUM2.pool.tag.uniq.t2c.CIMS.s13.bed 2095 PUM2.pool.tag.uniq.del.CIMS.s13.bed
This will keep only those with FDR<0.05. Significant sites will also be sorted by FDR, then by the number of tags with mutations, and then by the total number of overlapping tags. One might try different thresholds to get a balance of sensitivity/specificity (e.g. as judged from motif enrichment).
Note 1: Another parameter that might be useful to improve signal to noise is m/k (i.g., $8/$7 in awk)
Note 2: The number of significant CIMS might be slightly different when unique tags are provided in different orders (this could occur easily when multiple replicates are concatenated together). This is because the FDR estimation is based on permutation, which can change slightly depending on the order of tags.
Note 3: To examine enrichment of motif around CIMS:
awk '{print $1"\t"$2-10"\t"$3+10"\t"$4"\t"$5"\t"$6}' PUM2.pool.tag.uniq.t2c.CIMS.s13.bed > PUM2.pool.tag.uniq.sub.CIMS.s13.21nt.bed
#+/-10 around CIMS
This BED file could be used to extract sequences around CIMS (pay attention to the strand), and search for enrichment of specific motif (e.g., UGUANAUA for the case of PUM2) relative to the CIMS.
Download CIMS output files here (4 KB).
One can repeat these steps for the other types of mutations (i.e. deletions, insertions and general substitutions). This is particularly relevant when one works with a new RBP, and it is unknown which type of mutations will be caused by cross linking.
Motif enrichment
The enrichment of motif sites (when the motif is known) around CIMS or CITS provides a quantitative measure of the signal to noise ratio. The plot below shows the proportion of sites with the PUM2 binding UGUANAUA motif starting at each position relative to CIMS (T-C substitutions). This analysis shows U7 as the major crosslink sites in this dataset, which is consistent with the analysis of the original study (Hafner et al. 2010).